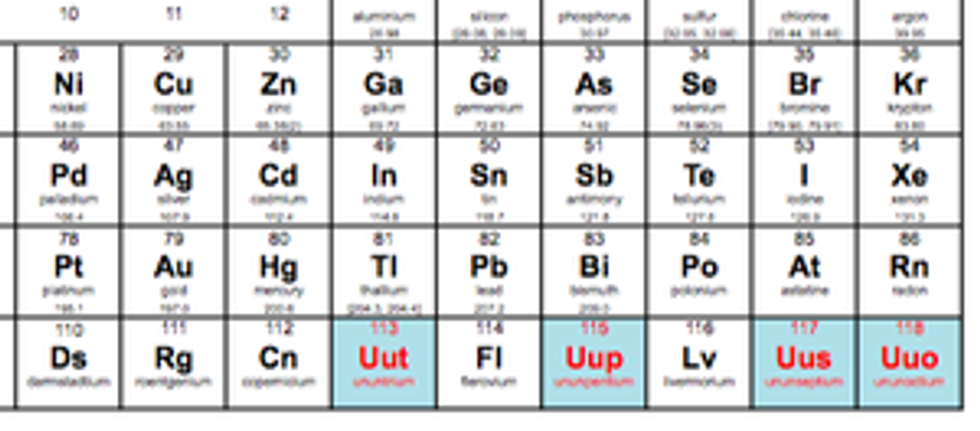
Just when you thought you had the periodic table memorized, four new elements had to be added to make chemistry even more confusing than it already is. Scientists in Japan, Russia, and America have discovered these new elements, which have completed the seventh row of the table, according to the International Union of Pure and Applied Chemistry (IUPAC).
However, these poor new elements have not been officially named yet and do not have symbols either. Their temporary names are Ununtrium, (Uut or element 113), Ununpentium (Uup, element 115), Ununseptium (Uus, element 117), and Ununoctium (Uuo, element 118). Don’t worry little elements, you’ll get some names soon, because the IUPAC has officially initiated the process of formalizing names and symbols for you.
These elements were not “easy” to discover either, unlike previous elements such as oxygen. It took over 100 scientists split up into four teams distributed across three countries to discover these new elements. These new elements are considered to be super-heavy elements due to their atomic number being greater than 100. If you know anything about chemistry, you know that the higher the atomic number, the more protons are in the nuclei, making it a more complex and rare element. The fact that four new elements were discovered is rather impressive and exciting for all chemists out there.
Is this where the periodic table stops growing? No way! Though this does mark the completion of the seventh row, chemists are still trying to make elements 119 and 120 in order to begin an eighth row. There is no evidence of success yet, according to Paul Karol, chair of the IUPAC Joint Working Party, but this doesn’t mean they're giving up. The world of scientists and chemists are intrigued and in awe of the fact the seventh row has finally been completed. This leaves many questions to be asked on what these new discoveries mean for chemistry in the near future.