Analysis of Cholesterol in Egg Yolk and Walnut Lipids
Introduction
In this experiment, the amount of cholesterol in egg yolk and walnut lipids was determined. Although the procedures are slightly different depending on what organism’s cholesterol levels are being measured, the process is still very useful as measurement of cholesterol levels and are an important part in measuring someone’s health.
Methods
Egg Yolk
To begin, an egg was cracked and the yolk separated from the white; it was then weighed. 30mL of methanol was mixed into the yolk thoroughly, and afterward 60mL of chloroform was added and mixed into the solution thoroughly. This mixture was then filtered into a beaker and the precipitate was discarded. 22mL of 1% NaCl solution was added to the filtrate and stirred; the top (aqueous) layer was drawn off with a pipette and discarded. To remove any dissolved water, a small amount of anhydrous sodium sulfate was added to the mixture, which was then decanted into a beaker. The volume of this solution was measured, and then a few grains of hydroquinone were added. 5mL of this solution was transferred to a pre-weighed vial and placed in a fume hood.
Walnut
About 2g of walnuts were weighed and then crushed in a mortar and pestle with a 10mL solution of chloroform and methanol in a 2:1 ratio. This mixture was then transferred to a large test tube, which was corked and placed in a 50-degree Celsius water bath for 10 minutes. After the 10 minutes, the mixture was filtered through filter paper; the filtrate then had 4mL of 1% NaCl solution added to it. The upper layer was then drawn off with a Pasteur pipette. Anhydrous sodium sulfate was added to the solution, which was then decanted into a beaker. The volume of this solution was then measured. The solution did not have enough to transfer both 5mL of the solution into a pre-weighed vial and store more, so all of the solution was transferred into the pre-weighed vial.
Cholesterol Measurement
To measure the cholesterol content of the egg yolk lipids and the walnut lipids, a spectrophotometer was used; a base reading was measured in a spectrophotometer with a blank of chloroform, several concentrations of cholesterol were then measured, and finally the lipid extracts of the walnut and egg yolk were measured. To prepare these samples for the spectrophotometer, .4mL of each respective substance was transferred into a test tube along with 4mL of Liebermann-Burchard reagent, then vortexed and let sit in a hot water bath for 10 minutes.
Results
For the egg yolk, we found the total amount of lipids in the egg yolk to be 28mL.
For the walnut, the total amount of lipids was about 3mL.
Cholesterol Measurement
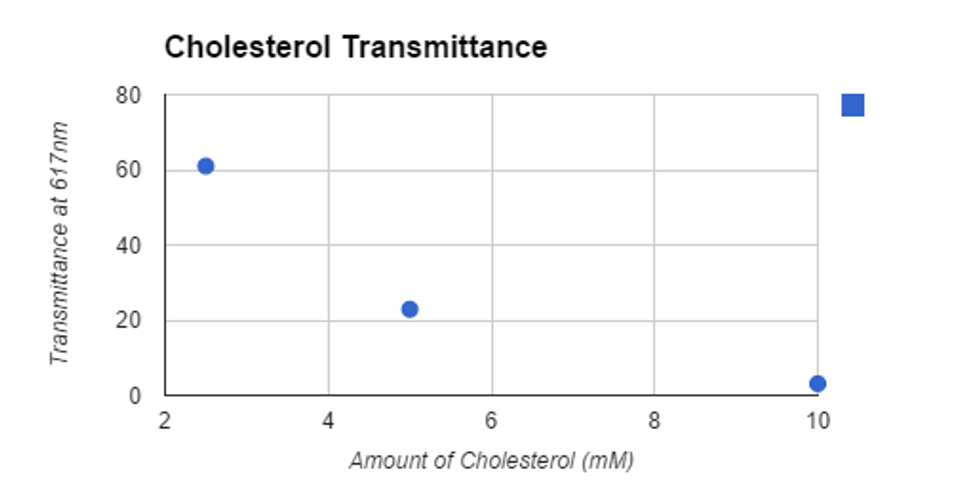
Tube | .4mL of: | Transmittance |
1 (blank) | chloroform | 100% |
2 | 2.5mM cholesterol | 61% |
3 | 5mM cholesterol | 22.9% |
4 | 10mM cholesterol | 3.1% |
5 | Egg yolk extract | 6.5% |
6 | Walnut lipid extract | 100% |
There is 28mL of lipid in the egg yolk.
About 8.5mM cholesterol concentration in the lipid extract— this was found by using the transmittance of the egg yolk extract and finding where on the line of best fit its complimentary y-value would fall; this gives an accurate approximation of the level of cholesterol in the egg yolk.
Discussion
The egg yolk was found to have a much higher lipid and cholesterol concentration than the walnuts. This was predicted— an egg yolk is nutrients for a developing chicken fetus, and thus, I expected it to have much more lipids in it than walnuts. Also, walnuts are a fairly lowfat food, so a high lipid concentration wouldn’t have made very much sense.
Analysis of Fats by H-NMR
Introduction
H-NMR spectroscopy is an important tool in organic chemistry and related disciplines; primarily it is used for determining the structure of organic molecules when only a molecular formula is known. However, it can also be used to check for purity in samples; for example, in this experiment the purity of two unknown samples of olive oil was tested by H-NMR spectroscopy to determine if the samples were polluted with vegetable oil.
Methods
Two samples of olive oil were used in the part of the experiment done by my group (this was a class experiment, with each group testing two samples each): one sample was an unknown and the other was a sample that was 70% vegetable oil with 30% olive oil.
Results
a | a’ | b | c | d | e | f | g | h+i | |
100% olive oil | 1.35 | -- | 9.18 | 1.64 | 1.50 | .888 | .127 | .604 | 1.008 |
100% veg. oil | .869 | -- | 5.338 | 1.107 | 1.104 | .615 | .393 | .414 | 1.000 |
30% OO/ 70% VO | .9659 | .0737 | 6.159 | 1.093 | .1162 | .6720 | .3448 | .4540 | 1.00 |
50/50 | 1.386 | .2885 | 9.285 | 1.521 | 1.644 | 1.000 | .3755 | .6759 | 1.343 |
70% OO/ 30% VO | 1.416 | .0988 | 9.725 | 1.488 | 1.662 | 1.000 | .2637 | .6822 | 1.247 |
Unknown 1 | 1.368 | .0226 | 9.286 | 1.709 | 1.529 | .9201 | 1.107 | .6247 | 1.000 |
Unknown 2 | .9595 | .1510 | 6.575 | 1.178 | 1.163 | .7116 | .2930 | .4744 | 1.000 |
To eventually find the compositions of the unknowns, the total number of fatty acids is first found
Fatty acids = He/2 = .7116/2 = .3558
The number of unsaturated fatty acids is found next
Unsaturated fatty acids = Hd/4 = 1.1631/4 = .2908
The percent unsaturated is then calculated = Unsaturated fatty acids/total fatty acids
.2908/.3558 = .6743 x 100 = 81.73% unsaturated fat in Unknown 2
100% - 67.43% = 18.17% saturated fat in Unknown 2
To find the percent of polyunsaturated fats, a special formula is used
(Hf - 2*Ha’/3)/He x 100% = (.2930 - ⅔ x .1510) / .7116 x 100% = 27.03% polyunsaturated fats in Unknown 2
To find monounsaturated fats, the number of polyunsaturated fats is subtracted from the total unsaturated fats → 81.73% - 27.03% = 54.7% monounsaturated fats in Unknown 2
Unknown 1 Stats
Total Fatty Acids = .46005
Percent Saturated FA = 16.92%
Percent Monounsaturated FA = 73.06%
Percent Polyunsaturated FA = 10.02%
Unknown 2 Stats
Total Fatty Acids = .3558
Percent Saturated Fatty Acids = 18.17%
Percent Monounsaturated Fatty Acids = 54.7%
Percent Polyunsaturated Fatty Acids = 27.03%
Discussion
From these results, accurate conclusions can be drawn about the purity of the unknown samples. Unknown 1 contains 73.06% monounsaturated fats, meaning it is probably mostly olive oil, as olive oil has a relatively low amount of polyunsaturated fats while having a high number of monounsaturated fats. Unknown 2, on the other hand, is only about half monounsaturated fats, meaning it has definitely had vegetable oil added to it—this can be seen in its lower percentage of monounsaturated fats and a higher percentage of polyunsaturated fats. The ratios of olive oil to vegetable oil in each unknown is unable to be calculated, but the relative amounts can be seen from the amounts of monounsaturated fats.