The Grignard reaction is by far my most favorite reaction—combining organic compounds with halogens and metals. Its main ingredients are an organic halide (most commonly a bromide), magnesium metal, and an electrophile (electron-poor.) Its main application is to create carbon-carbon (C-C) bonds between two organic compounds to synthesize a larger compound. Why is this so appealing?
It's because there are few reactions with the capability of making a C-C bond, such as an aldol addition or condensation, or a Wittig reaction. Despite the merits of these reactions, the Grignard remain a popular choice. This is because making the prerequisite halide is much easier, and magnesium metal is easily obtained. When magnesium is added to a dissolved alkyl bromide, it inserts itself between the carbon and bromine atoms and forms the Grignard reagent.
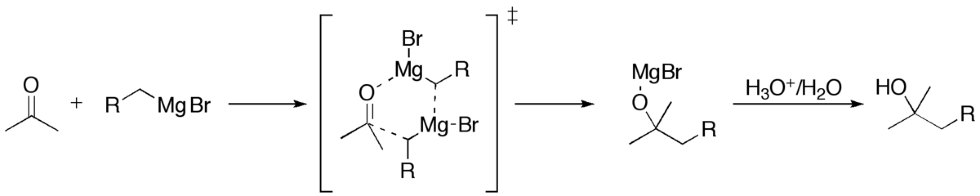
However, the real beauty of the reaction is shown when it can reach with an electrophilic carbon, as shown below.
Once the reaction proceeds, it's common practice to "quench" the reaction by adding a strong acid, such as hydrobromic acid. This enables the final product to be obtained (replace the -OMgBr with an -OH) to form an alcohol.
Despite being a very robust method to form C-C bonds, its Achilles heel is its sensitivity to impurities. For example, it will absolutely not work if the solution has water, as the magnesium will irreversibly react with any available water. Thus, the solvent containing the dissolved alkyl bromide needs to be meticulously dried such that no detectable amount of water can be present. This can be mitigated with molecular sieves, but the process can still be painstaking and time-consuming, as the Grignard reagent can even react with atmospheric water and significantly reduce yield.
The chemistry itself is just one reason why the Grignard is my favorite. I never knew that carbon could be directly bonded to a metal, given that carbanions tend to not be commonly found and that metals typically form ionic bonds with halogens or other oxidizing agents, such as oxygen. This reaction opened my eyes to the world of organometallic chemistry and its real-life applications.
One of my favorite chemists with the username NurdRage posted a video about making various types of alcohols for his future research projects through the Grignard reaction. This utilized the Grignard reaction several times to create heavy alcohols by stitching together long carbon chains to one electrophilic carbon that contains the eventual alcohol group. I was quite fascinated by just how well the reaction worked to give a decent sample of several alcohols that would go on to be used in his future experiments.